Zero-order kinetics in a CMBR
Environmental engineering
Problem statement
Lets consider 3 continuously mixed batch reactors (CMBRs) where a substance with initial concentration \(C_0\) decays with a zero-order rate constant \(k_0\). The parameters for each reactor are listed below:
- CMBR 1: \(C_0\) = 10 mg/L , \(k_0\) = 0.1 mg/Ls
- CMBR 2: \(C_0\) = 10 mg/L , \(k_0\) = 0.2 mg/Ls
- CMBR 3: \(C_0\) = 10 mg/L , \(k_0\) = 0.5 mg/Ls
- Plot the concentration in each reactor as a function of time.
- ¿What is the concentration of the substance in each reactor after 10 sec?
Solution
For a CMBR of constant volume \(V\), the concentration of a substance \(C\) that decays with a zero-order rate constant \(k_0\) can be modeled as follows:
\[ \dfrac{dC}{dt}V = k_0 V \tag{1}\]
Assuming that at time \(t=0\) the concentration of substance is \(C_0\), the previous ordinary differential equation (ODE) has the following analytical solution: \[ C(t) = C_o -k_0t \tag{2}\]
Note that, in this case, the concentration in the CMBR is independent of the reactor volume. Lets implement this last equation numerically through a function:
Our function receives the initial concentration C0, the zero-order decay rate constant k0, and the time. We can use the same function to model the 3 CMBRs.
Note that the time variable can be either a single value of an array of values. Thus, we can compute the evolution of the concentration in a CMBR as a function of time.
Plot of concentration
fig, ax = plt.subplots(figsize=(3,3)) # figure size
time = linspace(0, 20) # time in sec
k0 = [0.1, 0.2, 0.5] # zero-order decay rate constant (mg/Ls)
C0 = [ 10, 10, 10] # initial concentrations (mg/L)
for i, k in enumerate(k0):
Ct = conc(C0[i], k, time)
col = str(i*0.25)
ax.plot(time, Ct, color=col, label="$k_0$ = %s mg/Ls" % k)
ax.axvline(10, color='k', ls=':')
ax.set_xlabel("time [s]")
ax.set_ylabel("concentration [mg/L]")
ax.legend(edgecolor='k')
ax.grid()
plt.show()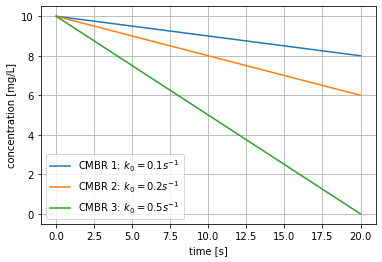
As expected from Equation 2, the concentration of each CMBR decays linearly as a function of time, with the slope being equal to \(k_0\). We additionally plot a vertical line at \(t=10\) s to visually inspect the concentration at this time.
Concentration after 10 sec
We can use our function considering 10 sec as the input time to compute the concentration of the substance.
Follow up questions
- From Equation 1, verify that the dimension of the zero-order constant is [\(ML^{-3}T^{-1}\)].
- Show that the time it takes a CMBR to reach half of the initial concentration \(t_{1/2} = C_0/2k_0\).
- Show that, for zero-order decay kinetics in a CMBR, the maximum simulation time is \(C_0/k_0\).
- Compute the concentraction of substance in the last reactor after 20 seconds.